Atomic Theory – Complete Guide on Atoms and Molecules
FREE Online Courses: Your Passport to Excellence - Start Now
Welcome to DataFlair Tutorial on Atomic Theory. Let us start learning the topics mentioned below:
What are Atoms and Molecules?
Atoms of most of the elements don’t possess an independent nature. They are responsible for the formation of molecules and ions. And then these molecules and ions join together and form an element we call matter.
This matter is tangible in nature. The chemical bonding of two or more atoms leads to the formation of a molecule. Multiple attractive forces are the reason for holding the atoms tight.
The smallest independent particle or compound in a matter is a molecule. It is very much capable of all properties of a substance. The Atoms are capable of joining with atoms of the same and the different elements in order to form a molecule.
The law of conservation says “mass can neither be created nor destroyed in a chemical reaction”. And Lavoisier came up with the Law of constant proportion.
Many compounds are made of two or more same elements but their proportion is the same no matter who the scientist is. This is what the law of constant proportion states.
An example is water, it follows a ratio of 1:8 of hydrogen and oxygen mass. The law of definite proportion is another name for this statement. The law by Proust states “in a chemical substance the elements are always present in definite proportions by mass”.
John Dalton’s Atomic Theory
John Dalton is an important name to remember in connection with the basic theory of the nature of matter. The scientist had to explain the concept above the laws and British Scientist Dalton stepped up for it.
The Greeks came up with the name “atom” and Dalton followed the law of chemical combination named atom the smallest particle.
Technology is evolving rapidly!
Stay updated with DataFlair on WhatsApp!!
This theory explains the connection between the law of conservation of mass and the law of definite proportions. 1808 was the turning point in his study as he was able to prove his atomic theory.
He came to the conclusion that all elements or compounds or a mixture is made up of the smallest particle – atom. The theory by him states certain points about atoms –
- All matter is made of very tiny particles called atoms.
- Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
- Atoms of a given element are identical in mass and chemical properties.
- Different elements atoms have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds.
- The relative number and kinds of atoms are constant in a given compound.
Dalton also became the first scientist for using element symbols for different formulas. It was mainly to define the quantity of the substance. Berzelius came up with the idea of using element names as symbols.
Before the name of the elements was after their originating location. An example is copper from Cyprus. In some cases, color played some role in naming the element like a gold element from yellow color.
International Union Of Pure And Applied Chemistry is the authority for approving element’s names. The English name is usually the first two letters of a symbol. The first letter of the elements is always a capital letter followed by a lowercase letter.
Molecules of Elements and Compounds
An element consists of molecules made up of the same type of atoms. Some examples are argon, helium, as they have only one type of atom. But the case is different in non-metals. The oxygen has 2 oxygen molecules becoming diatomic molecules. If another oxygen molecule adds up creating ozone.
The number of atoms in a particular molecule is atomicity. Metals and some other elements have a complex structure with indefinite bonding of large atoms. The molecule of a compound has atoms of different elements in definite proportions.
Ion
The metals and nonmetals have compounds that have charged species called ions. They may be negatively or positively charged. The positive ion is a cation and the negative ion is an anion.
An example is NaCl which has cation – na+ and anion – cl-. An atom group with ions becomes a polyatomic ion.
Writing Chemical Formulae
The symbolic representation of a compound composition is a chemical formula. Valency is the joining capacity of an element. Valency helps in finding out how one element will combine with another to form a chemical compound.
They are an important component of an atom. The chemical formula formation follows certain rules. They are –
- There must be a balance of valencies on an ion.
- The name of the metal is first, in case of metal and non-metal exits together in a compound.
- The polyatomic ion must be in a bracket before adding the ratio number. Like – Ca(Oh)2.
- This indicated two or more same ions in the formula.
Atomic mass, Molecular mass, and Formula unit mass
Dalton also spoke about atomic mass in his theory. Each element according to him has an atomic mass feature. One atomic mass unit is 1/12 the mass of an atom of carbon-12. The carbon-12 is the criteria for finding the relative atomic masses of all elements.
The molecular mass is the total of atomic masses of atoms in a substance. This is the relative mass of a molecule written in an atomic mass unit.
The total of atomic masses of all atoms in a compound is the formula unit mass. It follows the same formula as molecular mass. And the word formula use is only in substances with ions.
Mole Concept
The mass of the molecules can indicate substance quantity. But in the case of a chemical reaction formula, a direct number of atoms or molecules in a reaction is present. Thus the quantity of the substance is a better reference than the mass.
A mole is a unit that acts as the quantity number having an equal mass to its molecule in grams. A single mole has a value of 6.022 ×10^23. This is a fixed value with valid experimentation.
This number honors Amedeo Avogadro, a scientist by calling himself, Avogadro Constant. This concept is commonly used for comparing masses of different elements. The carbon isotope has atomic mass 12 and the relative mass of other atoms comes from mass carbon-12.
The Avogadro constant remains 6.022 ×10^23 in 12g of carbon-12. The mole is the same as the number of particles in a substance and thus it is the same as atoms in carbon-12 of 12g.
Structure of The Atom
J Thomson is the scientist behind the discovery of electrons. The atom is never a simple or invisible particle and has subatomic particles – electrons. Goldstein before the idea of electrons managed to find a positively charged proton in an atom.
Protons have a charge that is opposite to electrons and are 2000 times larger in mass. The electron has a minus charge and is negligible. Thus the theory of atoms having protons and electrons in a mutual benefit became more relevant.
Proton forms the interior of an atom while electrons are in the outer layer. Dalton believed that the atoms are indivisible and indestructible but the presence of protons and electrons failed this idea.
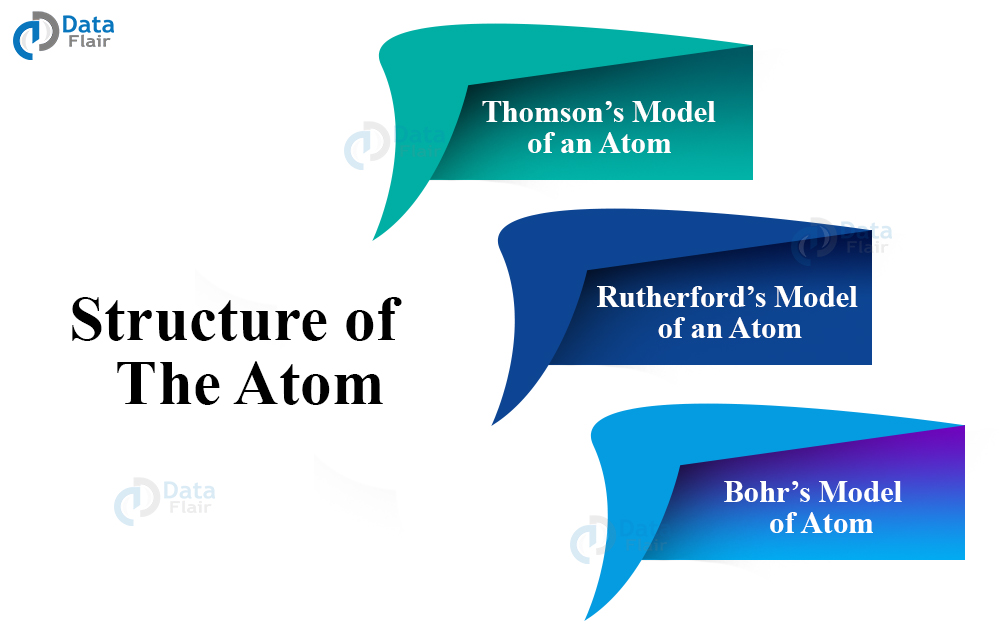
The arrangement of these elements became important to discover thus many scientists came up with different atomic theories and the first one was JJ Thomson. Let’s take a look at them –
Thomson’s Model of an Atom
Thomson was a British physicist with a Nobel prize in the same. This price was for his discovery of the presence of electrons in atoms. He said that the atom is similar to a watermelon. The protons are the edible part while the seeds were electrons in the charged sphere.
His conclusion to the theory was –
- The presence of a positively charged sphere in an atom where electrons live.
- Atoms are neutral electronically with protons and electrons having the same magnitude.
Thomson successfully proved that atoms are neutral but other scientists’ experiments did not align with this concept.
Rutherford’s Model of an Atom
The father of nuclear physics was Rutherford. His major contribution to physics was the discovery of the atom nucleus and his work in radioactivity. He was a Nobel prize winner for his gold foil experiment. The gold experiment studies the arrangement of electrons in an atom.
Fast-moving alpha helium ions particles fell on thin gold foil approx 100 atoms thick. There was a considerable amount of energy in these particles.
The expectation was to see the deflection of alpha particles by subatomic particles in the gold. But the deflection was high even when the alphas particles were heavier than protons.
The final observation was –
- The particles moved straight through the gold foil.
- Some particles saw small angle deflection.
- Every 12000 particles, there was a rebound.
Conclusion of the Rutherford Model of Atom was –
- There is a lot of empty space in an atom as many particles pass through the gold foil.
- There is a very less positive charge as many particles deflect from the path.
- The radius of the nucleus was 10^5 times less than the radius of an atom.
Features of Rutherford Model of Atom
- The nucleus is the positively charged centre of an atom with nearly all the mass.
- There is a well-defined orbit where electrons revolve around the nucleus.
- The nucleus size is quite smaller than the atom size.
Limitations of Rutherford’s Model of Atom
There were certain drawbacks to this theory. The orbit of an electron can not be stable and go under acceleration. The acceleration leads to energy release by charged particles. This will make electrons lose their energy and fall into the nucleus. Thus atoms become an unstable unit which is definitely not.
Bohr’s Model of Atom
Neil Bohr came up with another model to overcome the limitation of Rutherford’s model. His observation was –
- A discrete orbit exists inside an atom.
- The electrons do not release energy while revolving on this orbit.
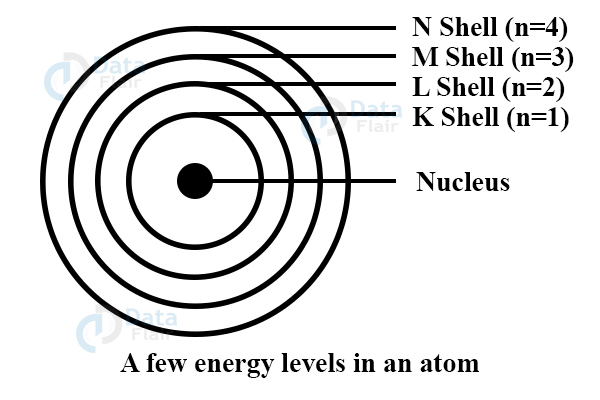
- These orbits are the energy levels having letters to represent them like k, m, 1, 2…
Rules for writing the number of electrons in different energy level –
- First orbit or k-shell = 2 ×1^2 = 2
- Second orbit or l-shell = 2 ×2^2 = 8
- Third orbit or m-shell = 2 × 3^2 = 18
- Fourth orbit or n-shell = 2 ×4^2 = 32
The ‘n’ is the max number of electrons in a shell. The maximum number of electrons in outermost orbit is 8. The filling of the shell is stepwise thus the inner shell must be full for electrons to enter the outer shell.
Neutrons
Chadwick is the scientist behind the discovery of neutrons. This particle in an atom does not have any charge and almost equal mass to protons. There are present atom nuclei except h20. The mass of an atom = masses of protons + masses of neutrons. The electrons have a negligible mass.
Subatomic Particles
Protons
- They carry a positive charge
- The charge is 1e = 1.602 × 10-19 (approx)
- Approx mass of a proton – 1.672 × 10-24
- Electrons are 1800 times lighter than them
- The atomic number of the element – total number of protons
Neutrons
- The mass of a neutron – 1.674×10-24
- They carry no charge
- Many isotopes have the same number of protons but a different number of neutrons
Electrons
- The charge is -1e = -1.602 × 10-19 (approx)
- Electron’s mass – 9.1 × 10-31
- The mass of an atom avoids electrons
Valency
The valence electrons are the electrons in the outer shell with a maximum capacity of 8 electrons. And an atom with a full outer shell has little chemical activity. Thus the combining valency is zero.
The combining capacity is the tendency of an atom to form molecules with other atoms of the same or another element. The outer shell has an octet which helps atoms react and achieve an octet in the outermost shell. The sharing, gaining, and losing of electrons facilitates this process.
This sharing and losing directly gives combining the capacity of an element or its valency. Hydrogen has only one electron in its outer layer and sling it makes its valency 1. In the case of a full outer shell, valency calculation changes.
Fluorine has 7 electrons in the outer layer that makes its valency 7. But gaining 1 electron is easier and thus the valency is 1. An atom of an element has a fixed combining capacity called valency.
Atomic Number
The number of protons in an atom is responsible for determining the atomic number. ‘Z’ denotes the atomic number. The atomic number is the same for atoms in the same element. The number of protons defines an element. Hydrogen has 1 proton thus z=1.
Mass Number
The atom mass particularly consists of protons and neutrons and they exist in the nucleus thus becoming nucleons. This proves that the mass of an atom is in the nucleus. The carbon has 6 protons and 6 neutrons and thus its mass is 12u.
The mass number is the total of neutrons and protons in the nucleus of an atom. The notation of an atom is –
- The atomic number
- The mass number
Isotopes
Many elements have atoms that have the same atomic number but different mass numbers. The hydrogen atom has three subatomic species – protium, deuterium, and tritium. The atomic number is the same for all three that is 1 but the mass number is 1,2 and 3 respectively.
The atoms of the same element with different mass numbers are isotopes. Thus hydrogen has three isotopes – protium, deuterium, and tritium. The mixture of isotopes exists as well but usually, it is a pure substance. The chemical properties of isotopes remain constant but the physical attribute changes.
Chlorine has two isotopic forms with 35u and 37u masses in a 3:1 ratio. The mass of any natural element acts as the average mass of all-natural element’s atoms.
In the case of isotope absence, the sum of protons and neutrons is the atom mass. But in its presence, % of isotopic forms decide the average mass.
Applications of Isotopes
The properties are the same for isotopes belonging to one element. Some of the isotopes have a special feature like –
- Uranium isotope helps as a fuel in nuclear reactors.
- Cobalt isotopes help in cancer treatment.
- Iodine isotopes treat goitre.
Isobars
Calcium and argon have 20 and 18 atomic numbers respectively. The electron number is different but their mass number is 40. Thus the number of nucleons is the same in both. Isobars are atoms of elements with the same mass number but different atomic numbers.
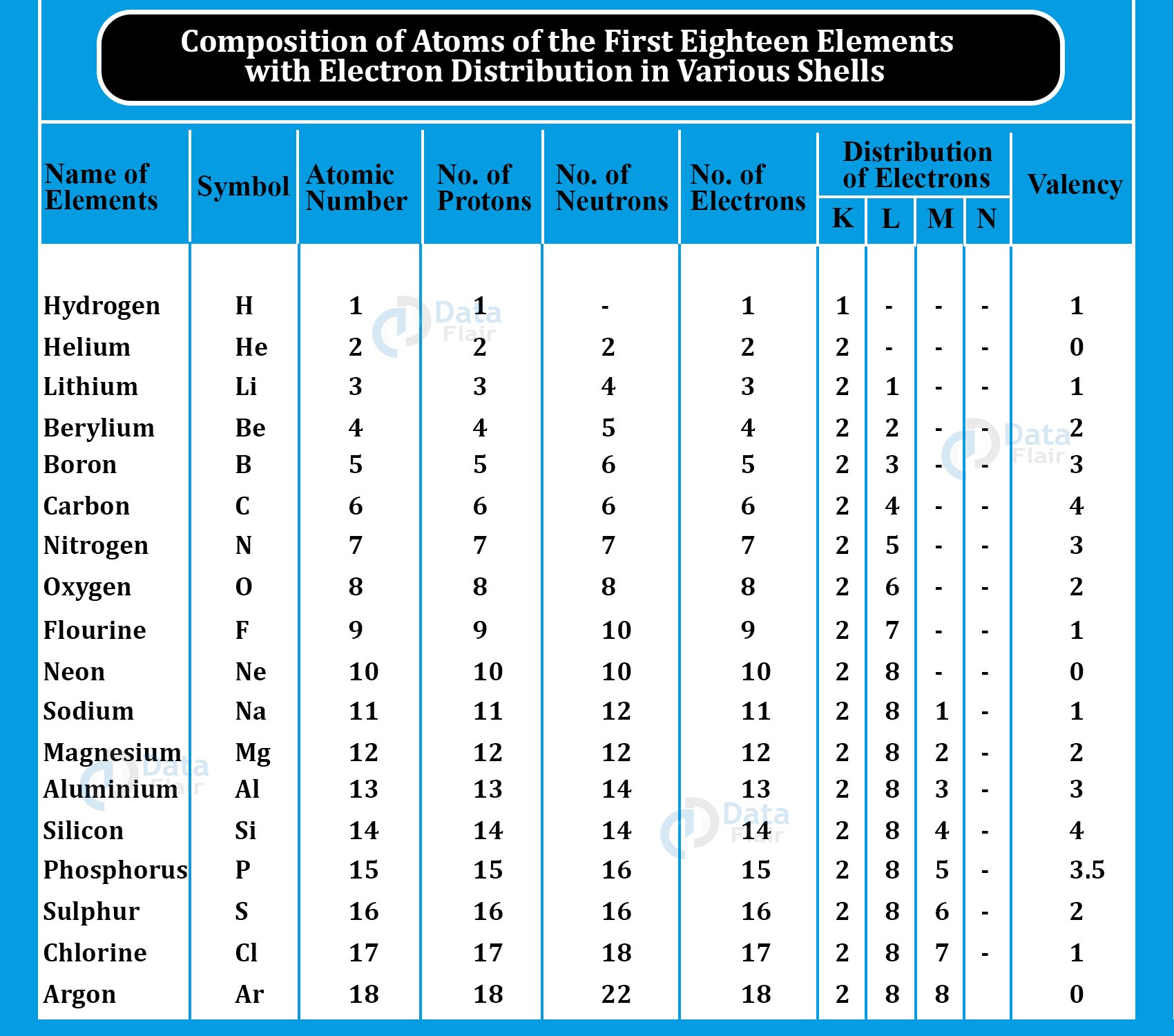
Atomic Structures of Some Elements
Hydrogen
An abundant hydrogen isotope is Protium. 1 is its mass and atomic number both. There is 1 proton and electron respectively and no neutron.
Carbon
12C and 13C are two carbon isotopes. 12C remains the most common. There are 6 protons, electrons, and neutrons each. The valence shell has 4 electrons. This allows chemical bonding more easily.
Oxygen
18O, 17O, and 16O are oxygen isotopes. Oxygen-16 is abundant in nature. It has 8 protons, electrons, and neutrons each. atomic number 8 while the mass number is 16. The valence shell has 6 out of 8 electrons.
Conclusion
This article was about atomic theory and atom structure at large. The most important parts were atom models and different terms around the atom structure. They are an important part of physics as well as general science. They are essential for knowing the existence of different matters in the world.
The UPSC Prelims General Study paper can have a question from this topic. This is because it comes under the basic science module. It is also important for UPSC Mains Physics as it is building a basic understanding of the subject.
Aspirants preparing for other competitive exams like SSC, RRB, and more can refer to it as well. All the UPSC candidates must go through this topic before appearing for the exams.
Your opinion matters
Please write your valuable feedback about DataFlair on Google